Achieving new milestones everyday
Countries of presence & supplies as of August 31, 2023
WHO prequalified medicines as of August 31, 2023
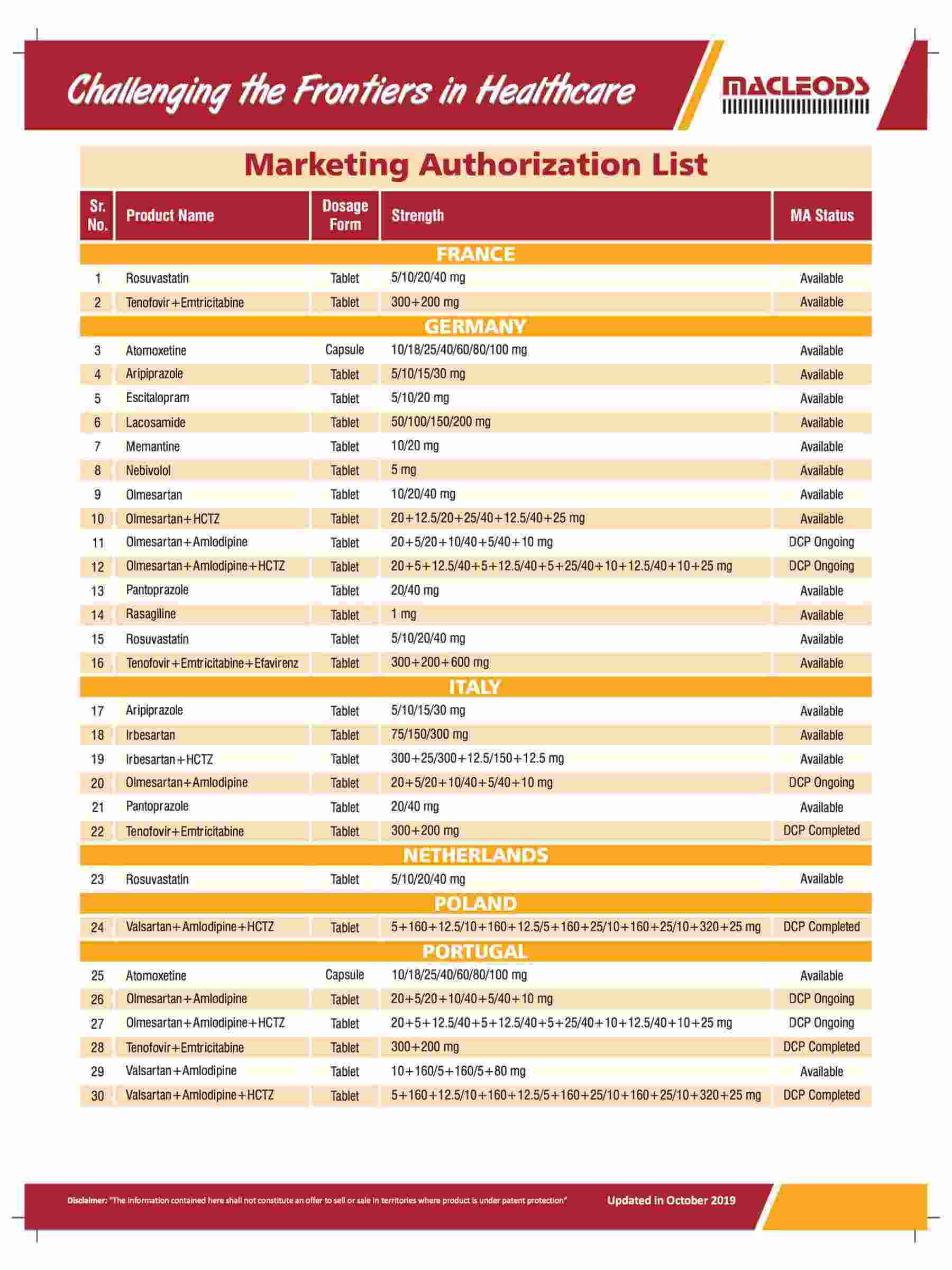
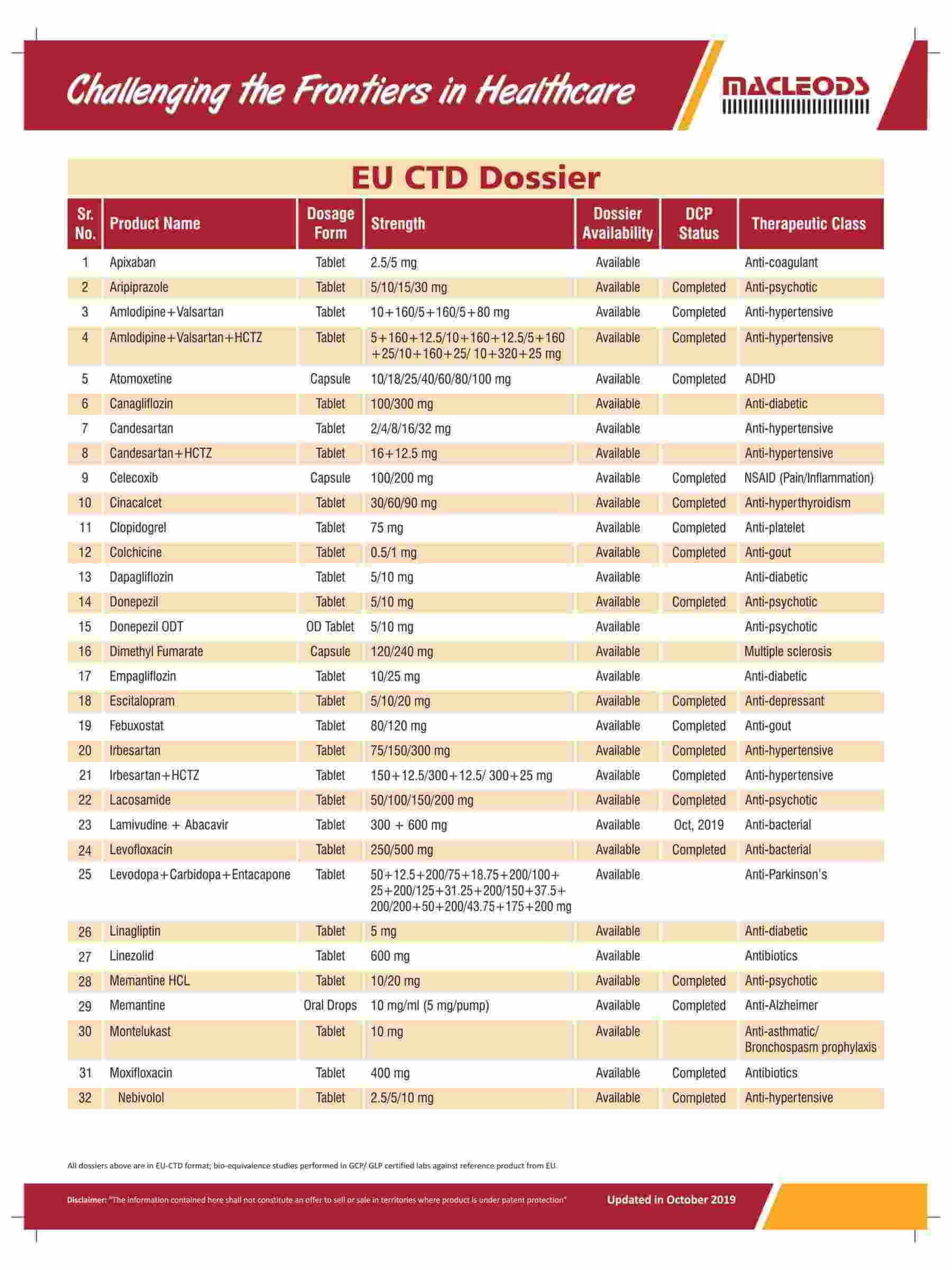
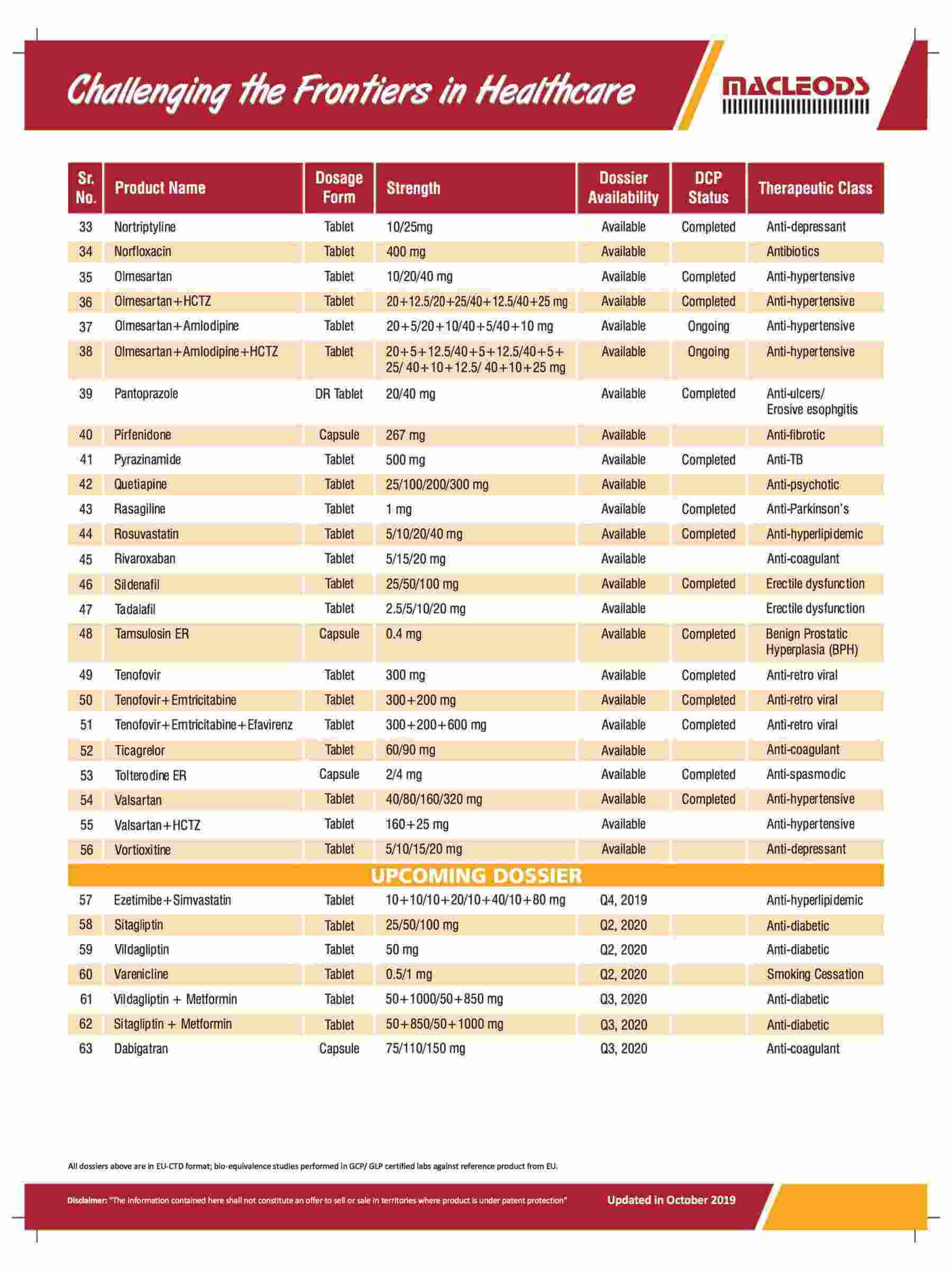
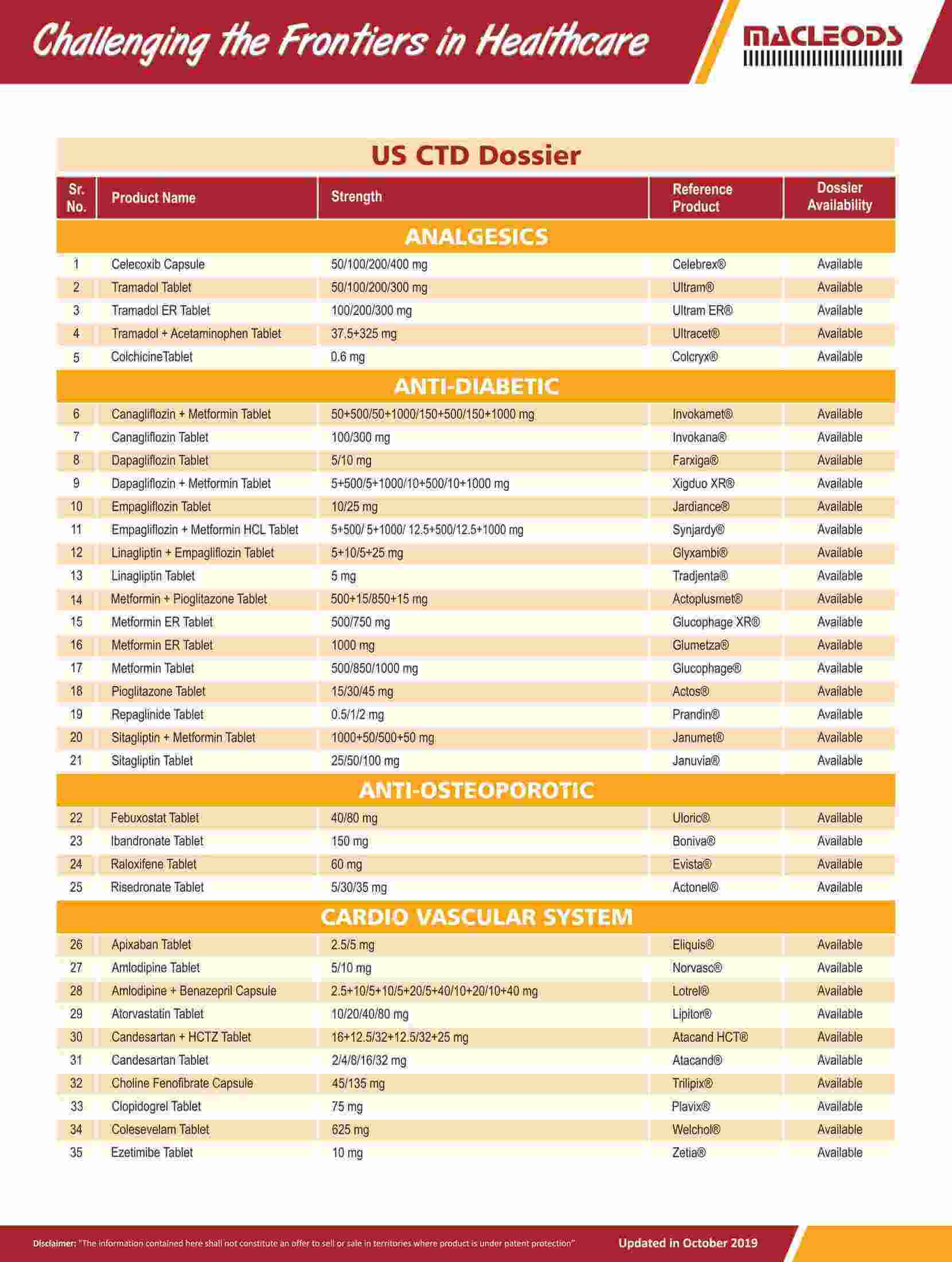
Product registrations globally as of August 31, 2023
R&D Centres as of August 31, 2023

Research & Development
Integral to our operations, are our R&D and bioequivalence centres. Our comprehensive R&D facility comprises of scientists and staff, who focus on the development of active pharmaceutical ingredients (APIs) and formulations, analytical development and bioequivalence studies. With 250 beds, this bioequivalence centre supports to conduct in-house bio equivalence studies.
Manufacturing
We take pride in our robust manufacturing operations and quality standards. We strive to meet these by focusing on achieving the highest benchmarks of hygiene, cleanliness and eliminating cross-contamination. Our manufacturing facilities are equipped to meet the mandatory prerequisites of Indian and various international regulatory bodies.

Our global footprint
Spotlighting our progress
- Global Offices
- Global Presence
- India : Corporate Office
- Kazakhstan : Branch Office
- Indonesia : Associate