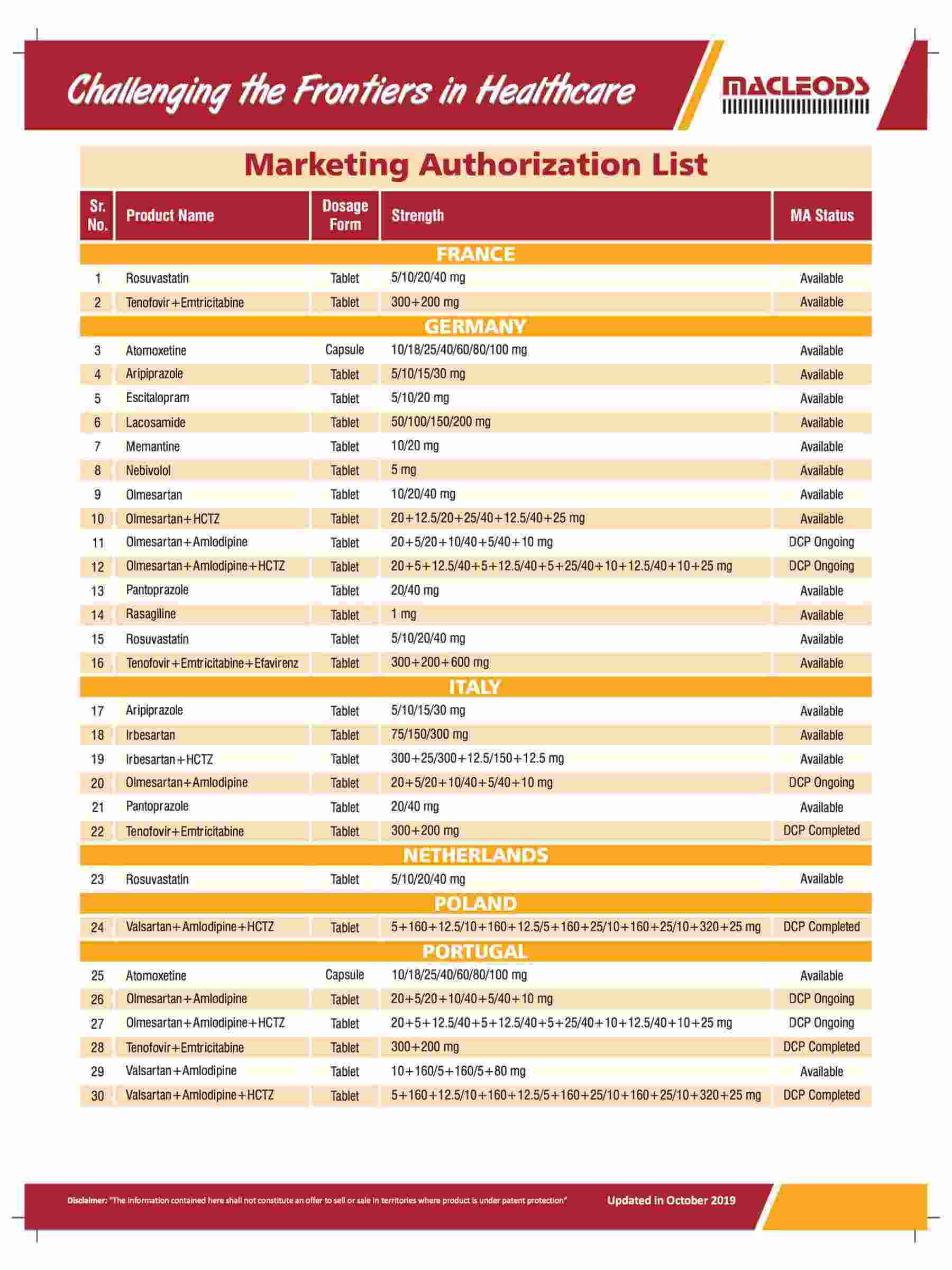
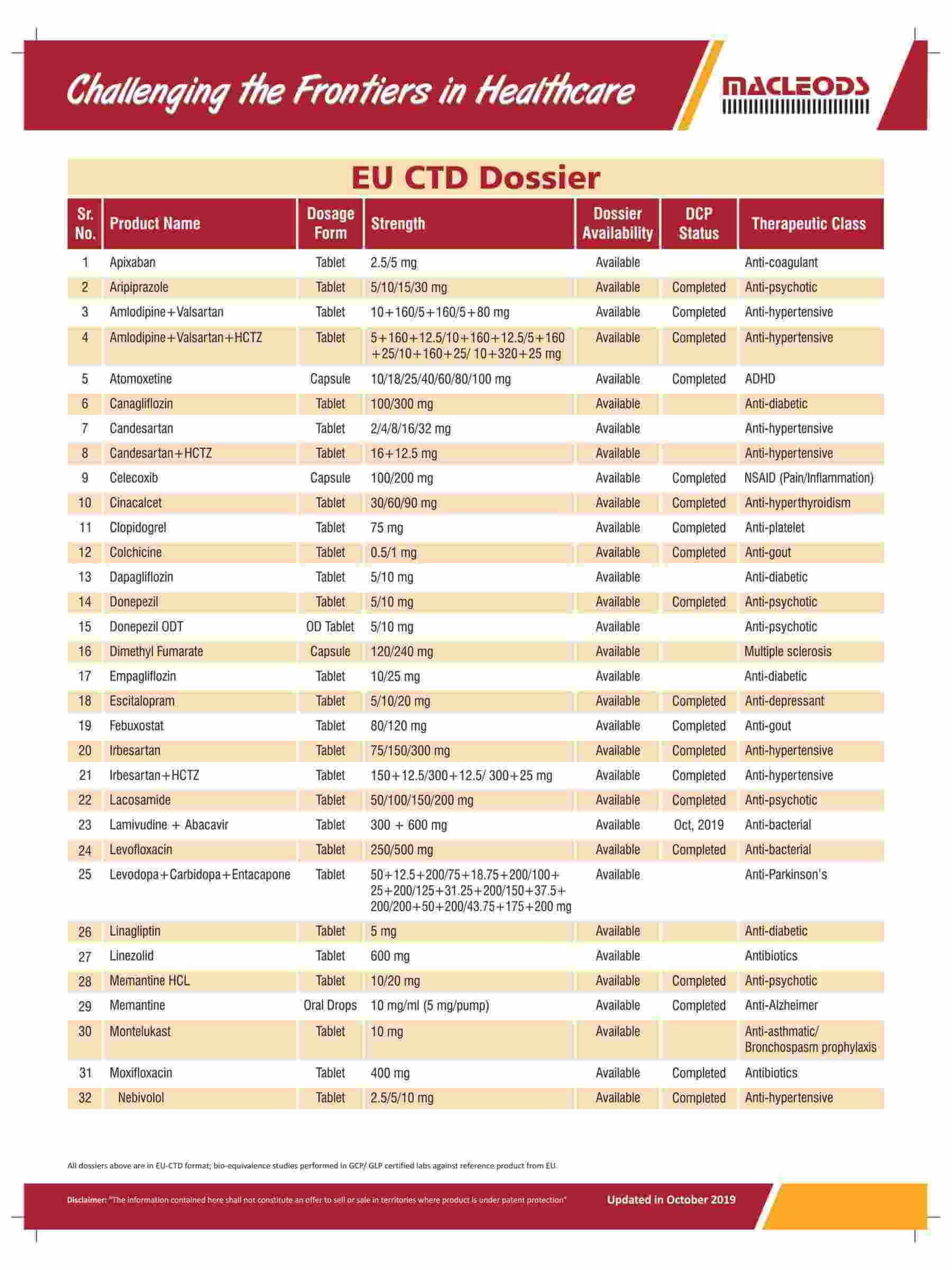
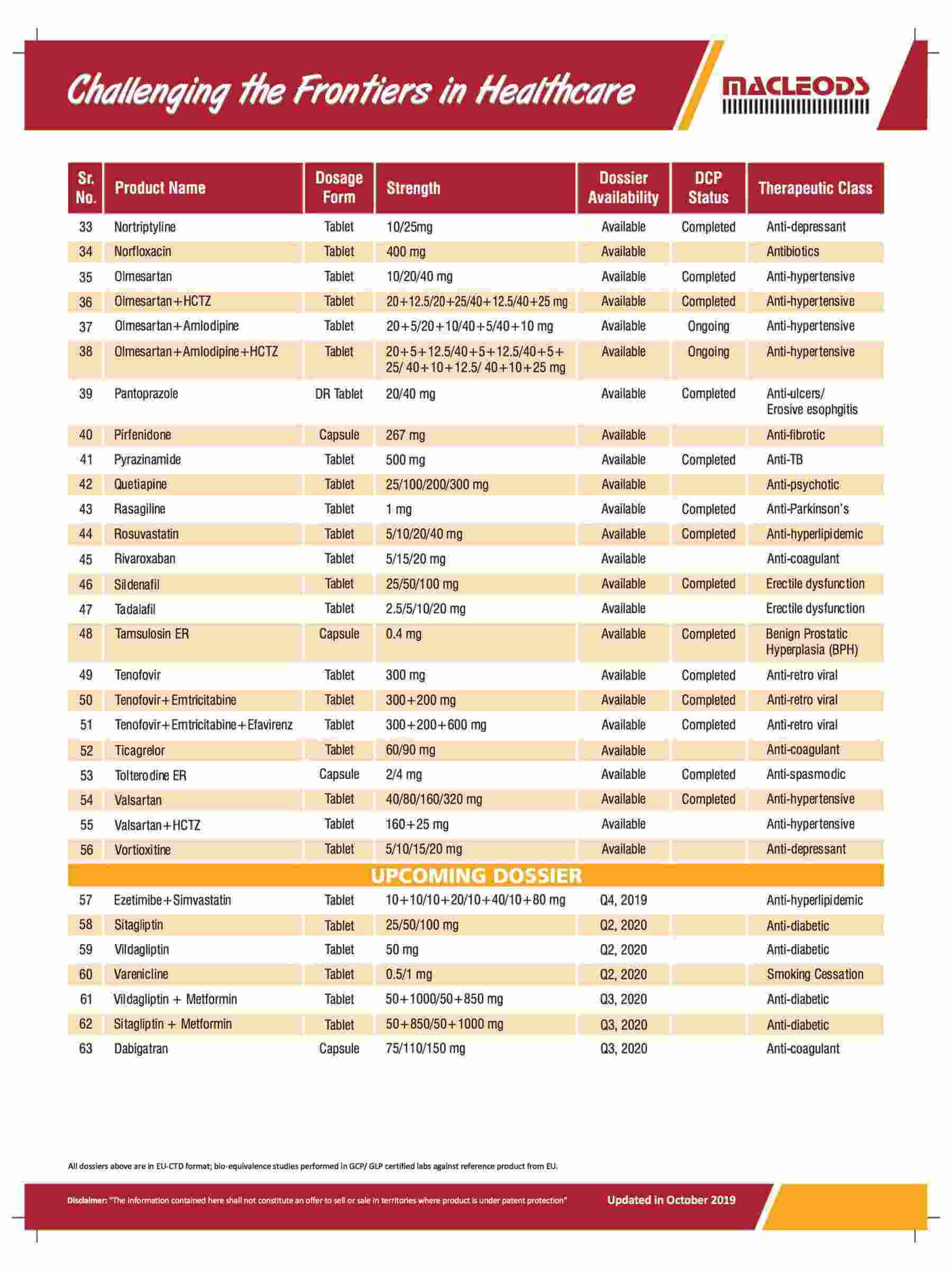
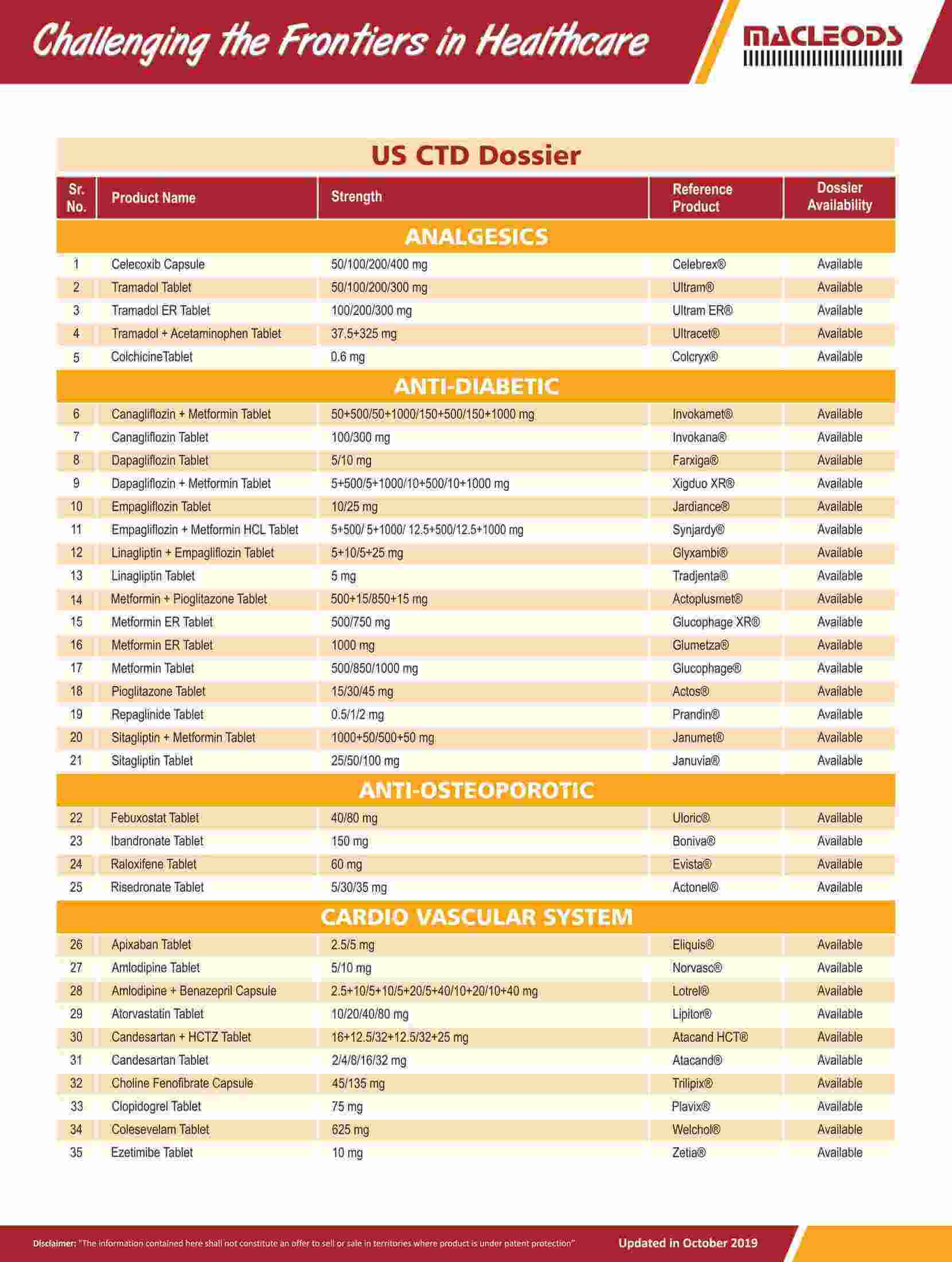
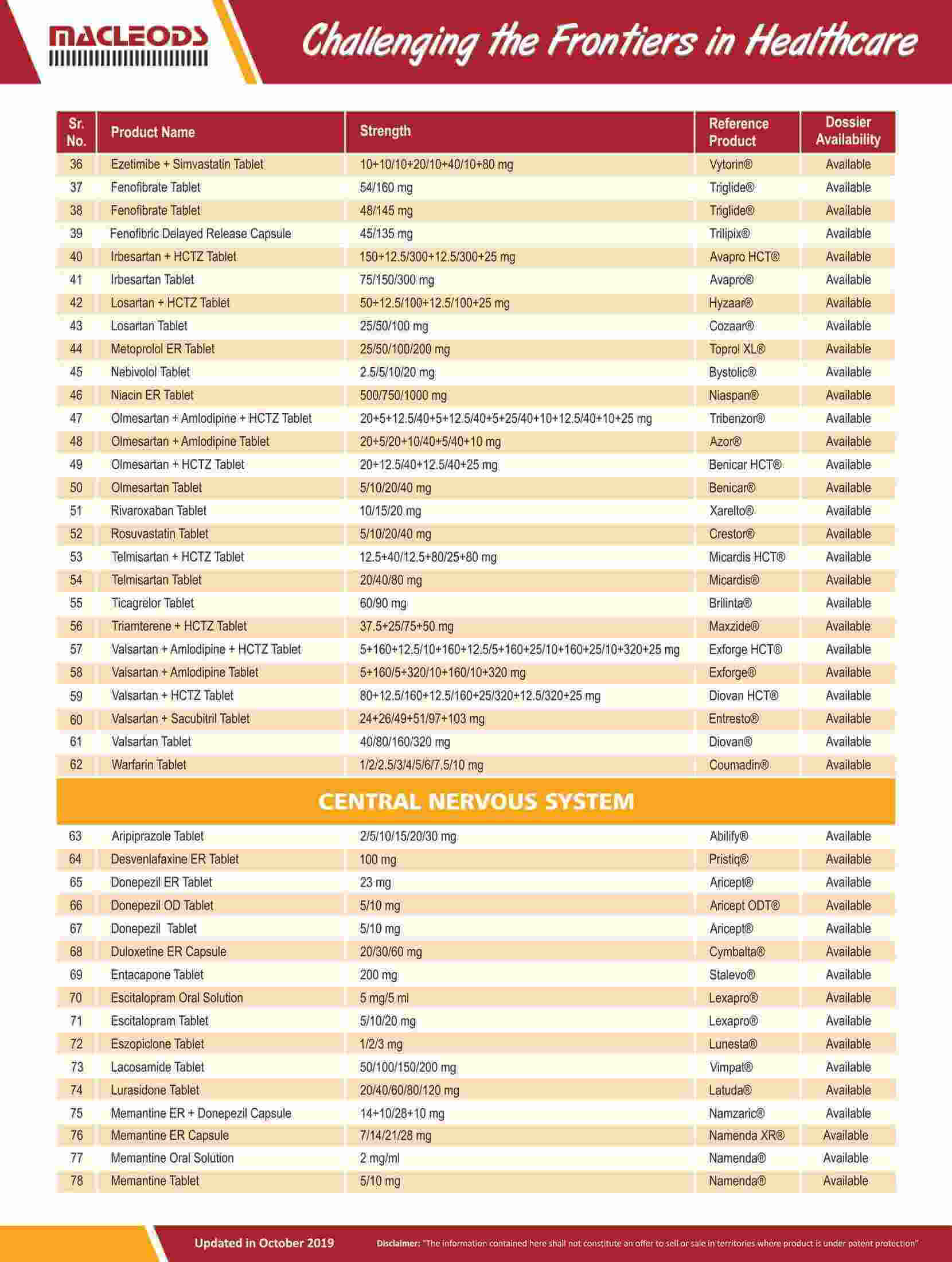
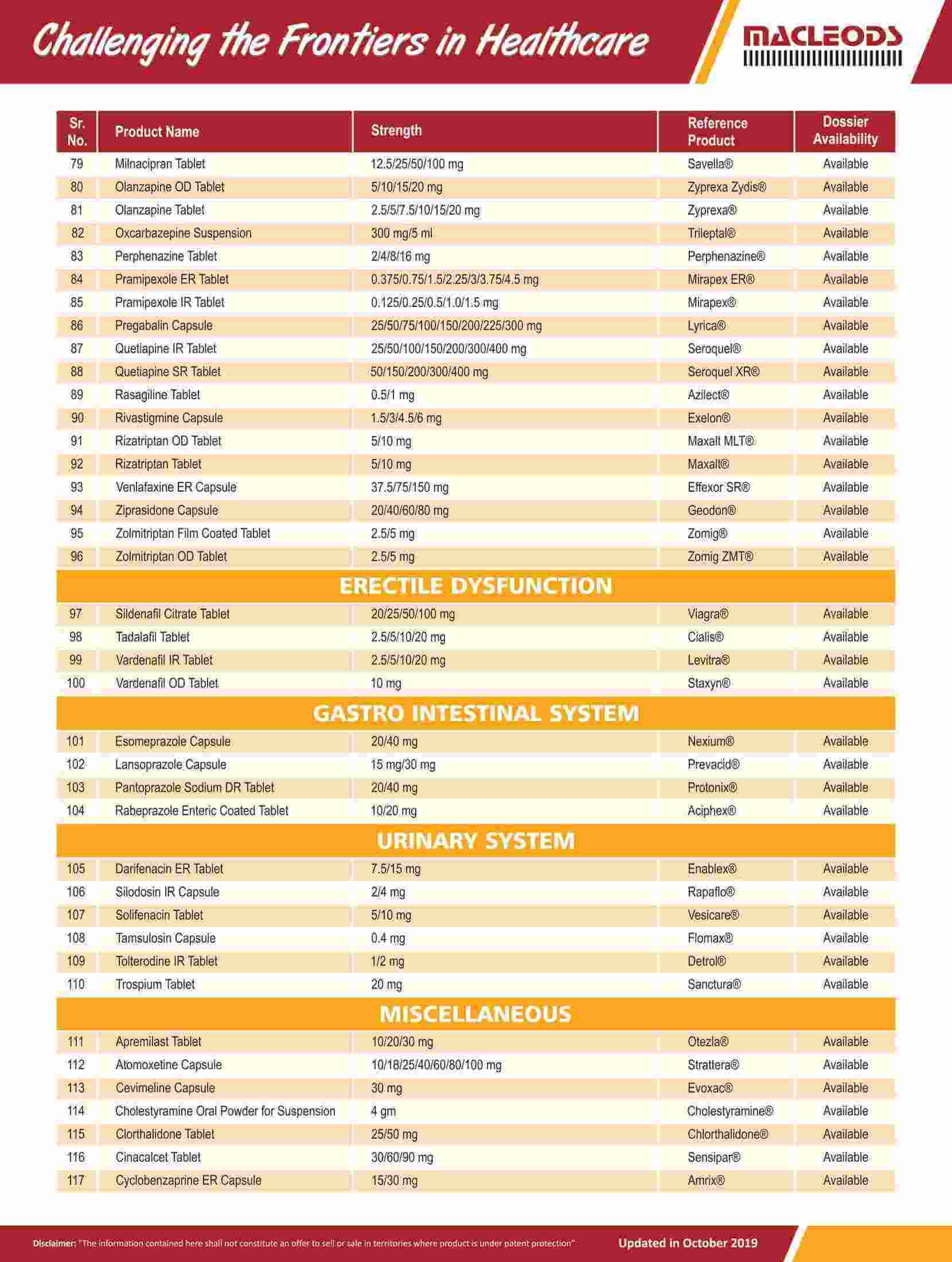
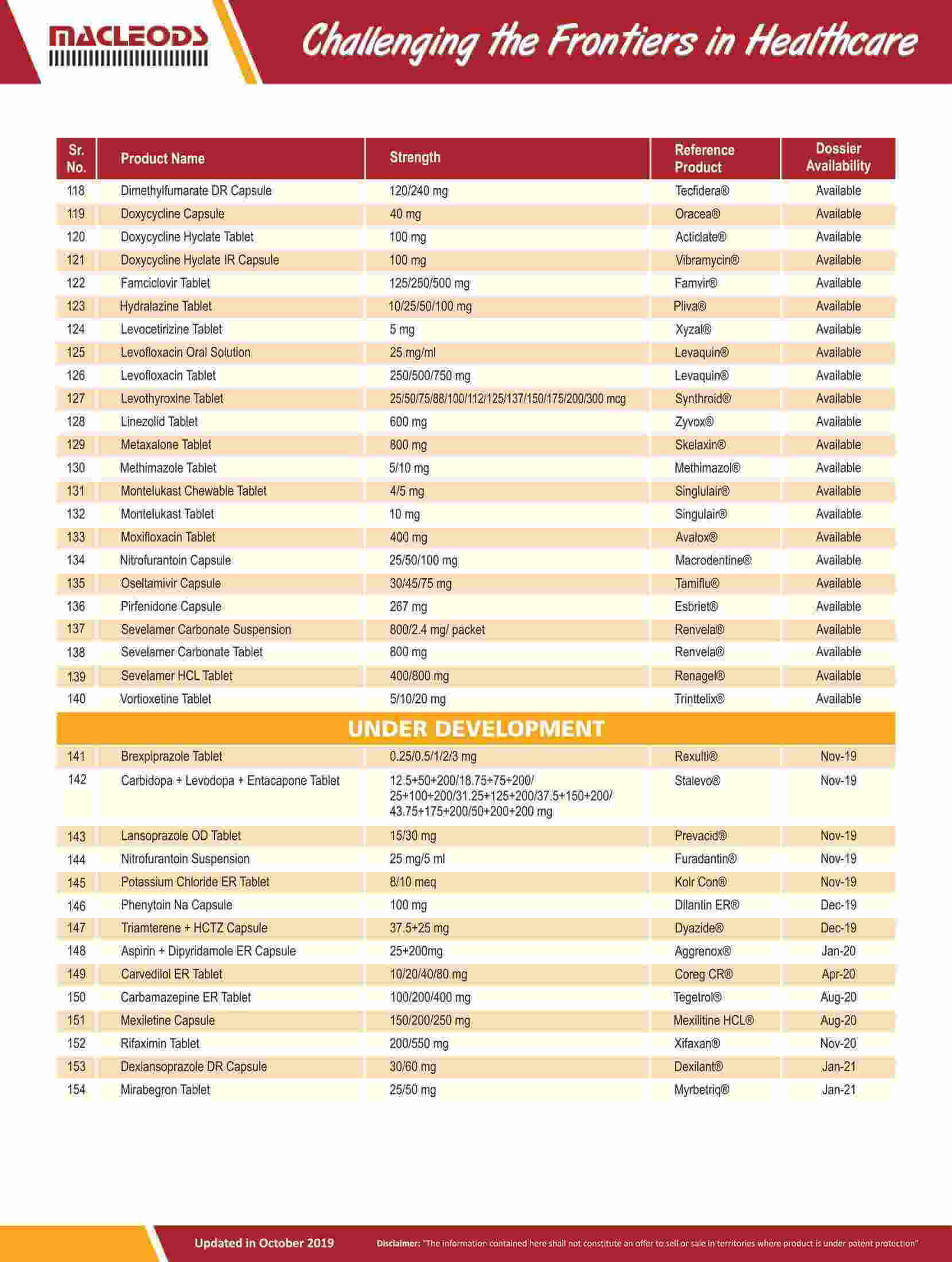
We are an R&D-driven company, which develops formulations across a range of therapies in multiple dosage forms.
As of August 31, 2023, we operated six DSIR-approved R&D centres for a range of development activities, including API, formulations research, and analytical development, led by a team of more than 1,900 employees including researchers and qualified scientists.
Our R&D-driven operations have resulted in a track record of product innovation and product launches
Our R&D teams focus on the development of pharmaceutical formulations, sophisticated characterisation of complex molecules, and product and process improvements to achieve better quality and efficacy for our existing products.
API development

Involved in the development of APIs, this division handles multi-step chemical synthesis involving varied reaction types including the use of chiral substrates. We comply with legal and regulatory requirements while working towards making the APIs scalable and cost-effective.
Product development

Through our R&D operations, we have been able to develop patented products including Rabemac DSR and file applications of a few other products. We have also developed products with new drug delivery systems to benefit patients, including Rabemac DSR (Rabeprazole plus Domperidone), Nexovas (Cilnidipine), Azithromycin (Zithrox), and Cefolac (Cefixime).
Analytical development

This department supports various forms of analytical development required for APIs and formulations. We use equipment such as Nuclear Magnetic Resonance, X-Ray Diffraction and Liquid Chromatography Mass Spectroscopy, among others.
Bioequivalence study centre

We established our bioequivalence centre in 2005 which is responsible for conducting studies for filing product registrations with various regulatory authorities. As of August 31, 2023, it held valid accreditation by CDSCO, DSIR-India and NABL. The centre is also successfully inspected by various Indian and international regulatory bodies and institutions such as USFDA, WHO Geneva, UK MHRA, MCC SA, ANVISA, SAHPRA & others. The centre is equipped to work across clinical, bioanalytical, pharmacokinetic functions and statistical, quality assurance and data protection, each integral to the organization.