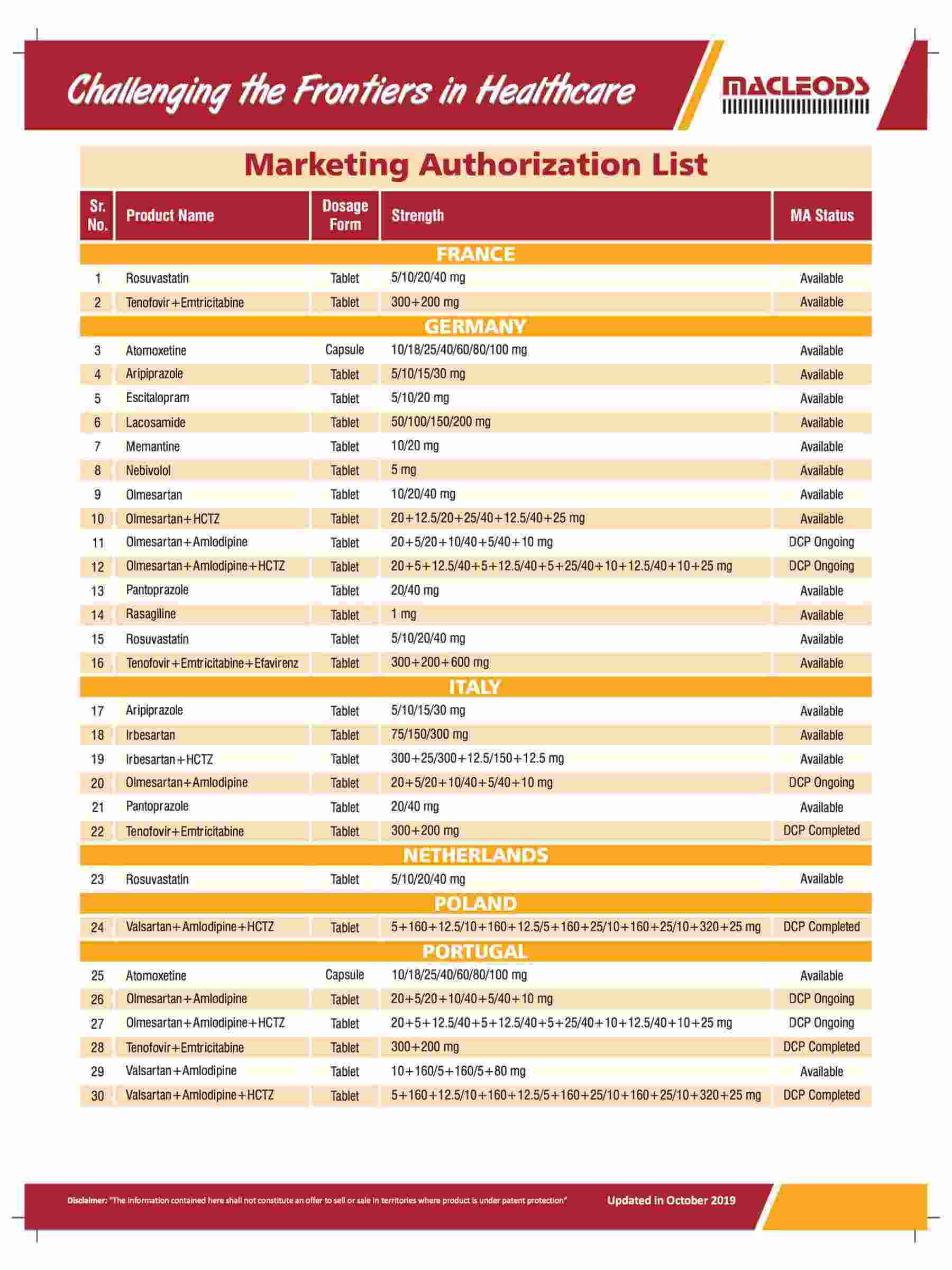
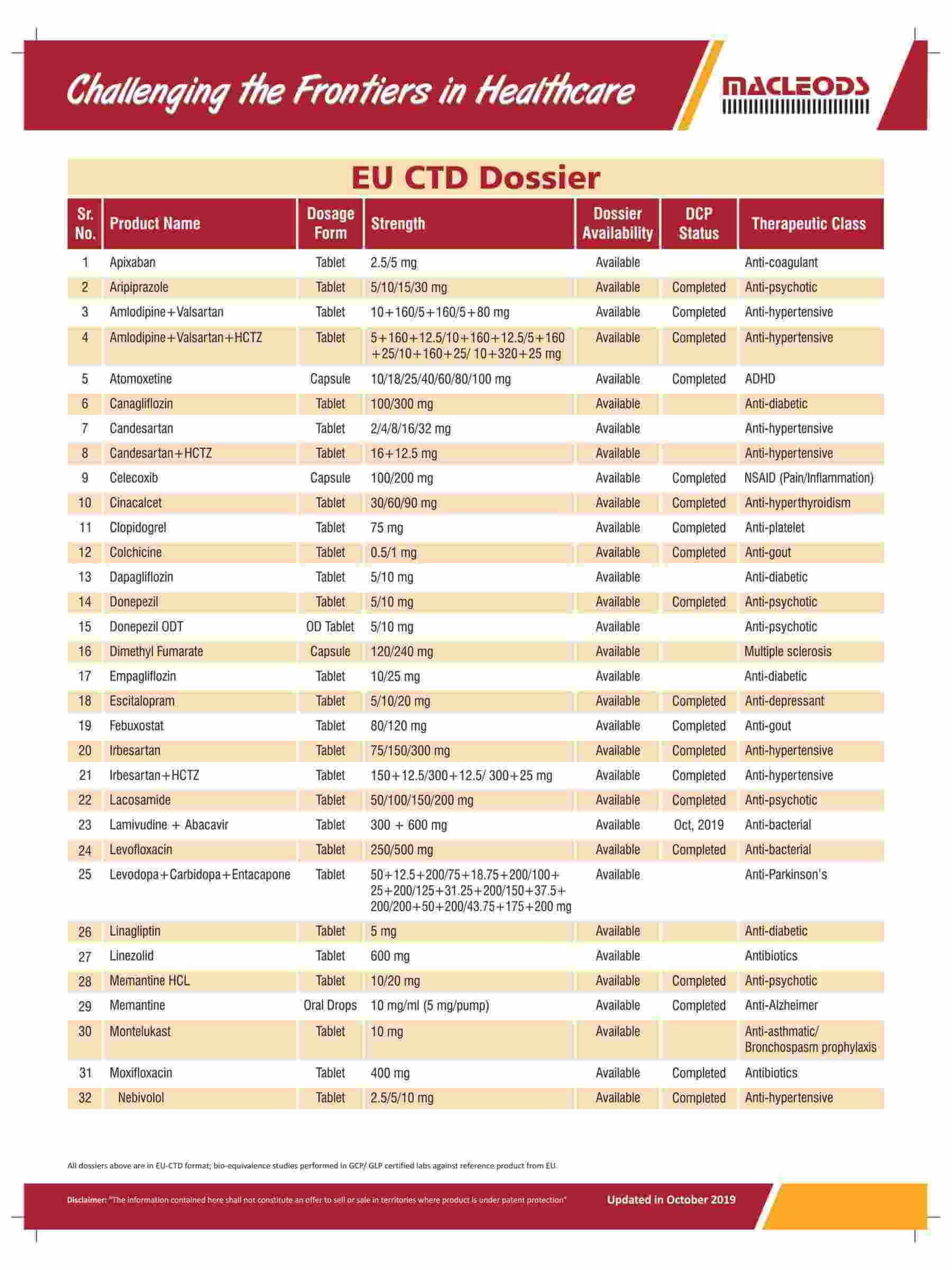
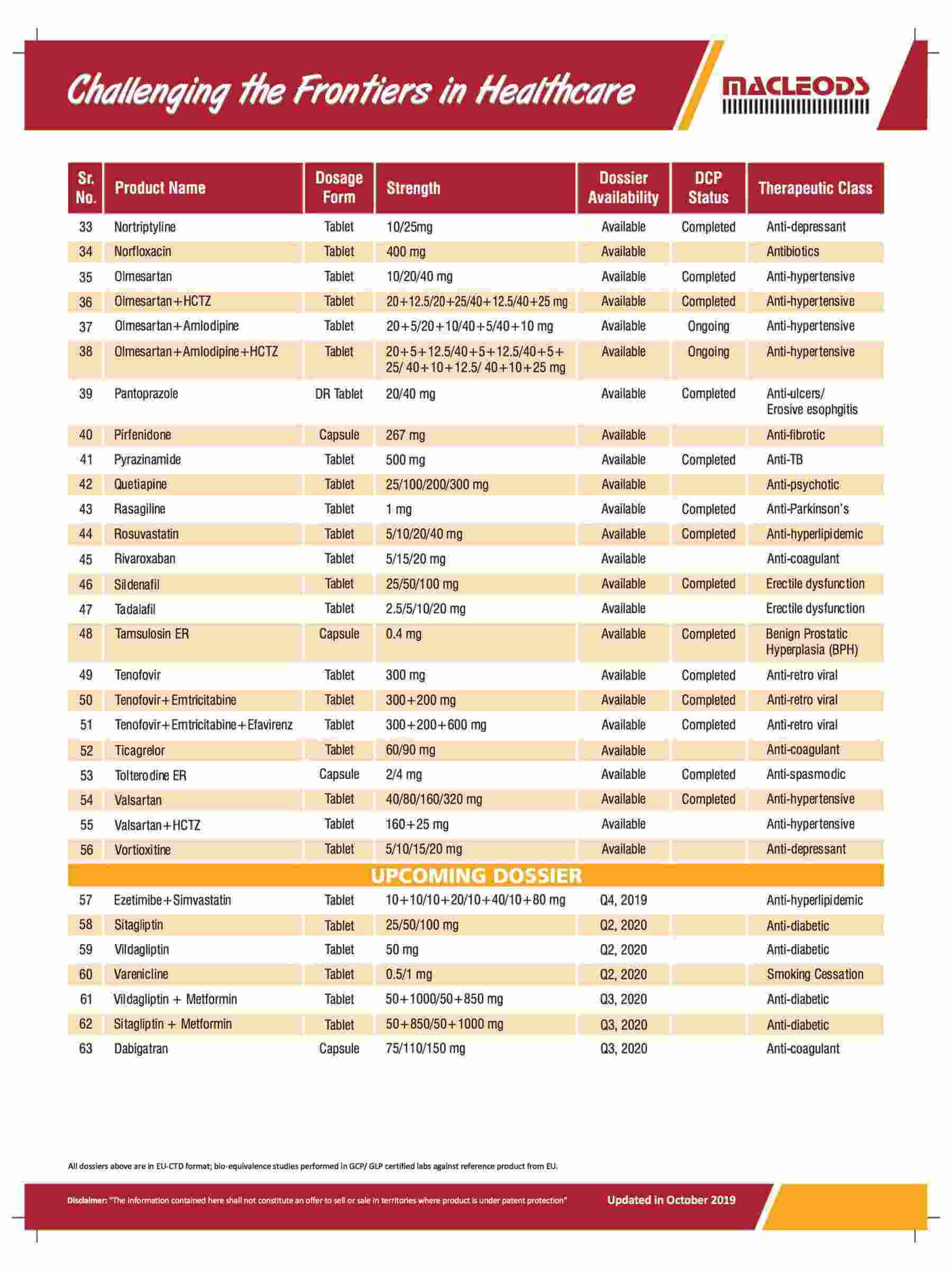
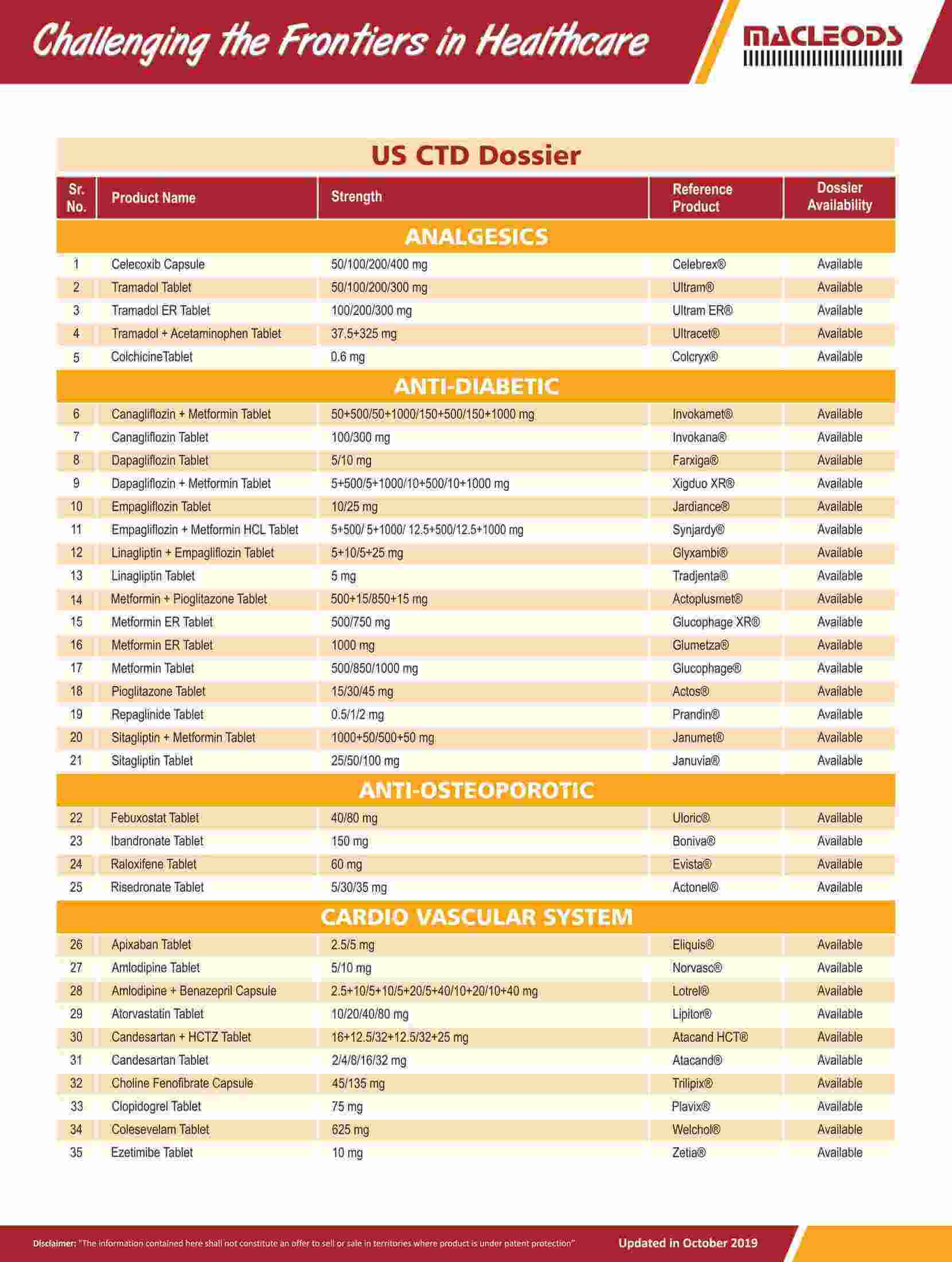
Diverse range of products that are prequalified by WHO and approved by USFDA and other international regulatory agencies
At Macleods, we have consolidated our presence in the essential medicines business by developing a range of anti-TB, anti-HIV and anti-malarial products, in addition to products for opportunistic infections and neglected tropical diseases.
Today, apart from having 60+ products prequalified by WHO, we also have to our credit, 30+ anti-TB products approved by the USFDA. Additionally, we offer a range of products that are SRA-approved (US/ EU) for supply to LMIC countries.
Our work
TB medicines
- 3HP for latent TB is a first of its kind and has been co-formulated as a fixed-dose combination of Rifapentine and Isoniazid. The course comprises of 36 tablets to be had for 12 weeks.
- We are one of the world’s largest producers of first/ second-line TB medicines.
HIV and advanced HIV medicines
- Our on-going focus includes ensuring economic efficiency through scale, reducing costs through chemistry improvements, ensuring savings through innovative packaging and logistical efficiency and developing better formulations (dispensing and heat-stable tablets).
- Our first-line fixed-dose combinations have been developed in line with WHO treatment guidelines.
- We are also working on several products for the management of Advanced HIV Disease.
Malaria medicines
- We offer a range of oral, rectal and injectable anti-malarial products in line with WHO guidelines.
- We have also developed products for seasonal malaria chemoprevention.
Our partnerships
In order to provide accessible healthcare to patients across the globe, we partner with several international government and non-government organisations. Because, we believe that by joining forces, we move one step closer towards tackling various ailments that afflict people worldwide .
International Donor-funded Agencies
The Global Fund
USAID – PEPFAR
PAHO
GDF
UN agencies (UNICEF, UNOPS, UNFPA)




UNICEF
Chemonics International
NGOs/ NPOs/ Wholesalers
MSF
MEG
IDA
Missionpharma
Imres
Amstelfharma






Médecins Sans Frontières (MSF)